|

![CCC(=O)[O-].[Na+]](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CCC%28%3DO%29%5BO-%5D.%5BNa%2B%5D&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
 |

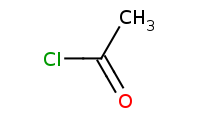
Note: Acid chlorides are one of the only carboxylic acid derivatives reactive enough to produce anhydrides by nucleophilic acylation
|
|
|


![CCC[O-].[Na+]](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CCC%5BO-%5D.%5BNa%2B%5D&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
 |

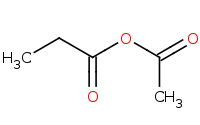
Note: Ester preparation from a reactive acid chloride, in this case with a base driven nucleophile
|
|
|

![CCC[O-].[Na+]](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CCC%5BO-%5D.%5BNa%2B%5D&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
 |
![CC(=O)[O-].[Na+]](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%28%3DO%29%5BO-%5D.%5BNa%2B%5D&width=200&height=125&arrowdesc=&extraImageSetting=amap)

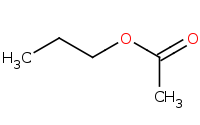
Note: Ester preparation from a reactive anhydride. Note the leftover 'leaving group' from the anhydride.


|
|
|

![CCC[O-].[Na+]](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CCC%5BO-%5D.%5BNa%2B%5D&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
 |
![CC(=O)[O-].[Na+]](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%28%3DO%29%5BO-%5D.%5BNa%2B%5D&width=200&height=125&arrowdesc=&extraImageSetting=amap)

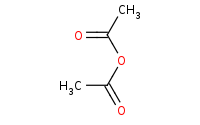
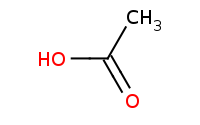
Warning: Base driven ester preparation will not work against carboxylic acids because acid-base reactions will occur first.
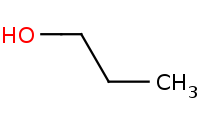

|
|
|
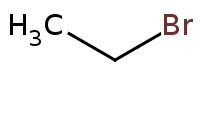

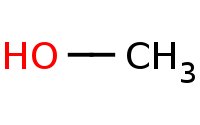

|
 |

Warning: No Reaction - Primary Alkyl Halide, Weak Nucleophile
Weak nucleophile excludes Sn2, E2.
Primary alkyl halide would yield unstable carbocation, so no Sn1 or E1 allowed either
|
|
|
Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%40H%5D%28C%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)



|
 |
OC](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40H%5D%28C%29OC&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: Sn1, E1 Competition - Secondary Alkyl Halide, Weak Nucleophile
OC](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%40H%5D%28C%29OC&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: Racemization (loss of stereospecificity) by Sn1 (compare to Sn2).
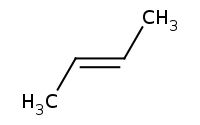

Note: Zaitsev rule preference for more highly substituted alkene, and preference for largest substituents oriented trans to one another
|
|
|
(C(C)C)Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%40%5D%28C%29%28C%28C%29C%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)



|
 |
(C(C)C)OC](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%40%5D%28C%29%28C%28C%29C%29OC&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: Sn1, E1 Competition - Tertiary Alkyl Halide, Weak Nucleophile
(C(C)C)OC](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%5D%28C%29%28C%28C%29C%29OC&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: Racemization (loss of stereospecificity) by Sn1 (compare to Sn2).
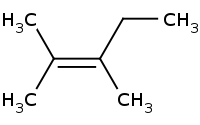

Note: Zaitsev rule preference for more highly substituted alkene
|
|
|
[C@H](C)Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%40H%5D%28C%29%5BC%40H%5D%28C%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)



|
 |
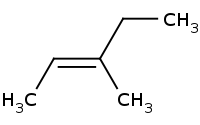

Note: E1 results in racemization, no stereospecificity. Always prefer the larger groups trans to each other
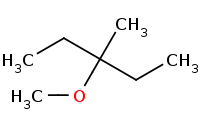

Note: Sn1 competition. Note that a carbocation rearrangement (hydride shift) occured
|
|
|
[C@@H](C)Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%40H%5D%28C%29%5BC%40%40H%5D%28C%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)



|
 |


Note: E1 results in racemization, no stereospecificity. Always prefer the larger groups trans to each other


Note: Sn1 competition. Note that a carbocation rearrangement (hydride shift) occured
|
|
|



|
 |
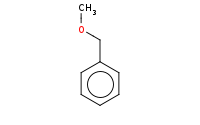

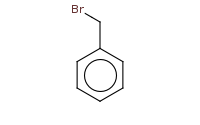
Note: Sn1 at primary benzylic halide. Apparent primary carbocation seems disfavored, but resonance at the benzylic position stabilizes it
|
|
|
|
|
|
(0.062 sec)
Link
|
|