|
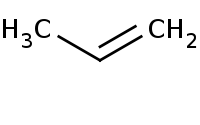

|
 |
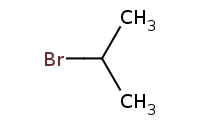

Note: HBr addition with Markovnikov regioselectivity based on carbocation intermediate preference for carbocation at the more highly substituted carbon
|
|
|
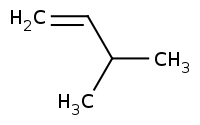

|
 |
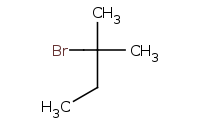

Caution: Carbocation intermediates can undergo rearrangements, such as the hydride shift in this example
|
|
|

|
 |
Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40H%5D%28c1ccccc1%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)

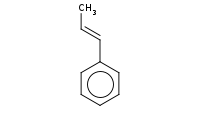
Note: Benzylic (and allylic) carbocations are relatively stable, conferring the regioselectivity in this reaction
Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%40H%5D%28c1ccccc1%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
|
|

|
 |
Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40H%5D%28C%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)

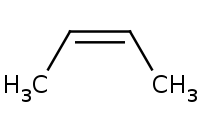
Note: Carbocation intermediate is achiral (planar), thus yielding both enantiomer products
Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%40H%5D%28C%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
|
|

|
 |
Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40H%5D%28C%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)

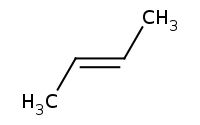
Note: Again, achiral carbocation intermediate yields both enantiomer products, regardless of starting material chirality
Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%5BC%40%40H%5D%28C%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
|
|
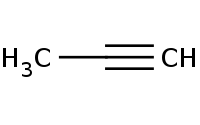

|
 |
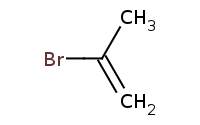

Note: HBr adds to alkyne π bonds similar to alkene addition with respective Markovnikov regioselectivity for terminal alkynes
|
|
|
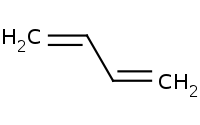

|
 |
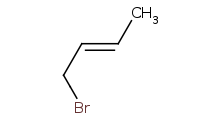

Note: Allylic carbocation intermediates have alternative resonance structures, resulting in the possibility of 1,4 additions beyond the "usual" 1,2 addition
Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D%28C%3DC%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40%40H%5D%28C%3DC%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
|
|
![C[C@@H]1C(O1)(C)C](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40%40H%5D1C%28O1%29%28C%29C&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
 |
(C)Br)O](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D%28C%28C%29%28C%29Br%29O&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: In contrast to 'base driven' epoxide opening like with organometallics, acid-catalyzed epoxide opening prefers the nucleophile (Br- in this case) to attack the more substituted site based on partial carbocation character
|
|
|
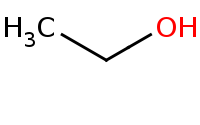

|
 |
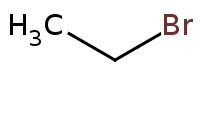

Note: Acid-catalyzed halide substitution (Sn2) of hydroxyl group.
|
|
|
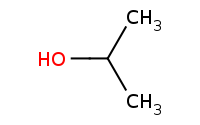

|
 |


Note: Acid-catalyzed halide substitution (Sn1) of hydroxyl group.
|
|
|
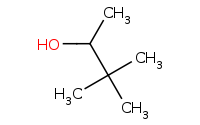

|
 |
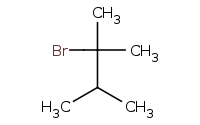

Caution: Acid-catalyzed halide substitution by Sn1 mechanism is susceptible to carbocation rearrangements
|
|
|
![C[C@H]1CO1](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D1CO1&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
 |
Br](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D%28CO%29Br&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: Acid-catalyzed epoxide opening. Note regioselective preference for more substituted side. Similar to Sn1/E1 selectivity, but no actual carbocation formed, thus still stereospecific
|
|
|
|
|
|
(0.066 sec)
Link
|
|