|
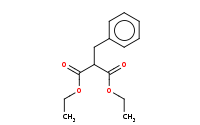

|

(hot, dilute)
 |
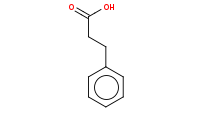

Note: Acid hydrolysis of esters to carboxylic acids. More importantly, once you produce and heat a carboxylic acid that is beta to another carbonyl (such as an ester), decarboxylation (loss of CO2) will readily occur via a 6-membered cyclic transition state. The net result of malonic ester synthesis is the apparent alkylation of acetic acid.
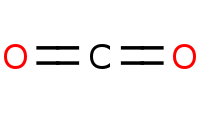

|
|
|
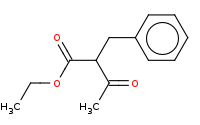

|

(hot, dilute)
 |
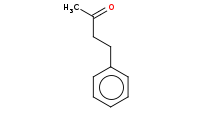

Note: Acid hydrolysis of the ester to a carboxylic acid which is beta to the ketone. Under heated conditions, this will readily decarboxylate to yield a net result that is the apparent alkylation of acetone.
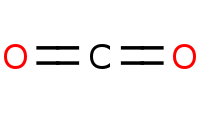

|
|
|

|

(hot, dilute)
 |
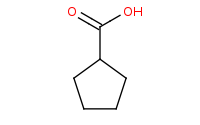

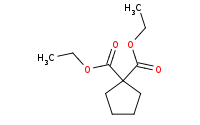
Note: Hot acid to yield decarboxylation results in a carboxylic acid substituent on a closed ring.
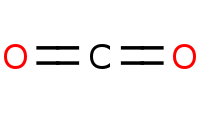

|
|
|
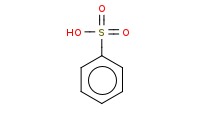

|

(hot, dilute)
 |
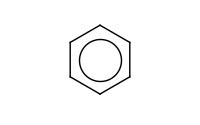

Note: Sulfonation of benzene is reversible with hot (steaming), dilute acid. This can make it useful as a temporary 'blocking group.'
|
|
|
(C)C)O](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CC%28C%29%28CCCCO%5BSi%5D%28C%29%28C%29C%29O&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|

(hot, dilute)
 |
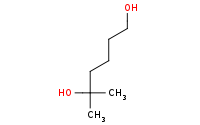

Note: Aqueous acid may also be used to deprotect silyl ethers
|
|
|
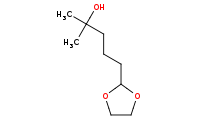

|

(hot, dilute)
 |

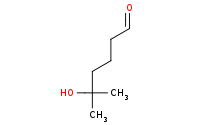
Note: Acetal protecting groups may subsequently be removed by aqueous acid hydrolysis

|
|
|
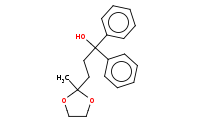

|

(hot, dilute)
 |


Note: Acetal protecting groups may subsequently be removed by aqueous acid hydrolysis after the highly reactive organometallic step has been completed
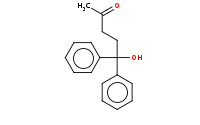

|
|
|
O](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D%28C%23N%29O&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|

(hot, dilute)
 |
O)O](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D%28C%28%3DO%29O%29O&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: Acid-catalyzed hydrolysis of nitriles to carboxylic acids


|
|
|
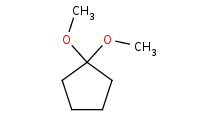

|

(hot, dilute)
 |
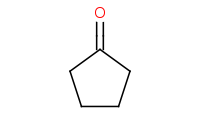

Note: Acid-catalyzed hydrolysis of an acetal back to the component carbonyl and alcohols. Excess H2O is assumed to drive the reaction equilibrium.
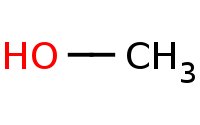



|
|
|
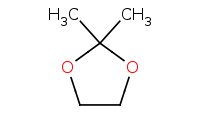

|

(hot, dilute)
 |


Note: Acid-catalyzed hydrolysis of a cyclic acetal back to component molecules
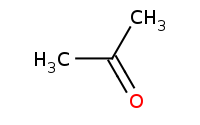

|
|
|
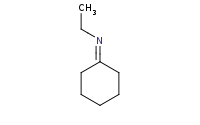

|

(hot, dilute)
 |
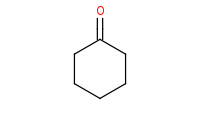

Note: Acid-catalyzed hydrolysis of an imine back to component molecules.
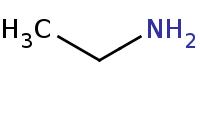

|
|
|
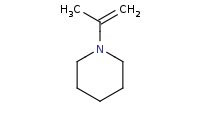

|

(hot, dilute)
 |
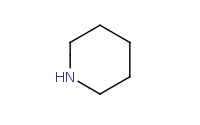

Note: Acid-catalyzed hydrolysis of an enamine back to component molecules.


|
|
|
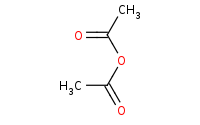

|

(hot, dilute)
 |
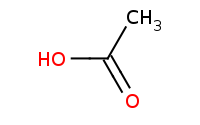

Note: Carboxylic acids can be prepared from more reactive derivatives like anhydrides by hydrolysis, in this case helped by acid catalysis.


|
|
|

|

(hot, dilute)
 |


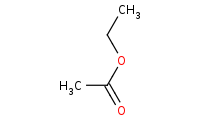
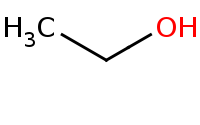
Note: Acid-catalyzed hydrolysis of an ester to a carboxylic acid, with excess water assumed to drive the reaction equilibrium. Virtually all carboxylic acid derivatives can be hydrolyzed to carboxylic acids with aqueous acid.

|
|
|

|

(hot, dilute)
 |


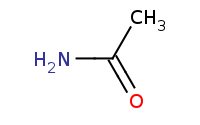
Note: Amides are among the least reactive carboxylic acid derivatives. One of the few reactions that can be completed is acid-catalyzed hydrolysis


|
|
|
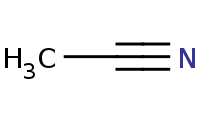

|

(hot, dilute)
 |


Note: Nitriles are counted among carboxylic acid derivatives in part because they can be hydrolyzed under acid conditions to yield carboxylic acids.


|
|
|

|

(hot, dilute)
 |


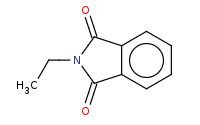
Note: The final step of Gabriel synthesis of a primary amine is hydrolysis of the phthalimide. This is comparable to hydrolysis of an amide to an amine and carboxylic acid.
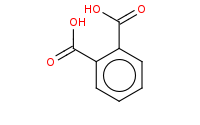

|
|
|
![c1ccc(cc1)[N+]#N.OS(=O)(=O)[O-]](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=c1ccc%28cc1%29%5BN%2B%5D%23N.OS%28%3DO%29%28%3DO%29%5BO-%5D&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|

(hot, dilute)
 |
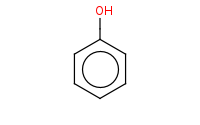

Note: Treating arenediazonium salts with aqueous acid results in substitution by water. This provides one of the only synthetic methods to produce phenols.
|
|
|
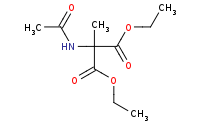

|

(hot, dilute)
 |
O)N](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40%40H%5D%28C%28%3DO%29O%29N&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: Amino-malonate derived synthesis of amino acids concludes with acid hydrolysis of the amide and esters, including decarboxylation of the resulting beta-di-carboxylic acid.
O)N](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D%28C%28%3DO%29O%29N&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|
|
|
N](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D%28C%23N%29N&width=200&height=125&arrowdesc=&extraImageSetting=amap)

|

(hot, dilute)
 |
O)N](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D%28C%28%3DO%29O%29N&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: Hydrolysis of the nitrile yields the carboxylic acid of the amino acid.
|
|
|
|
|
|
(0.066 sec)
Link
|
|