|
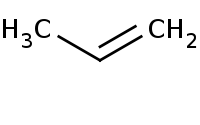

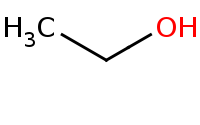

|
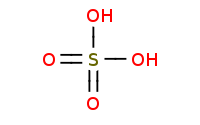
(catalyst)
 |
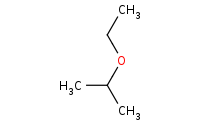

Note: Acid-catalyzed addition of alcohols works just the same as hydration, except the alcohol is the nucleophile instead of H2O
|
|
|
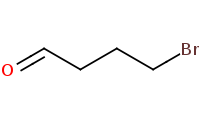

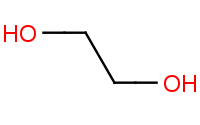

|

(catalyst)
 |
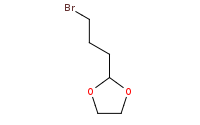

Note: Carbonyls may be protected with alcohols (especially diols) to form acetals
|
|
|
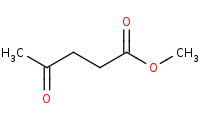



|

(catalyst)
 |
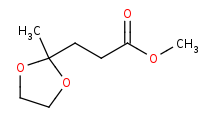

Note: Carbonyls like ketones are more reactive than esters which means they can also be selectively protected with alcohols to form acetals before any productive reaction with the ester occurs
|
|
|
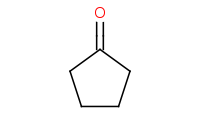

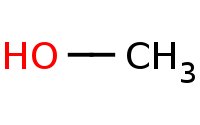

|

(catalyst)
 |
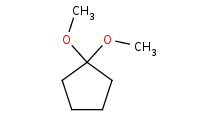

Note: Acid-catalyzed addition of an alcohol to a carbonyl. This initially yields a hemiacetal, but these are unstable so a second equivalent of alcohol will ultimately add to form a complete acetal. Note these are all reversible steps and generally the carbonyl form is more stable than the acetal form. Driving conditions, such as distillation of the H2O by-product, will be necessary to complete the reaction.
|
|
|


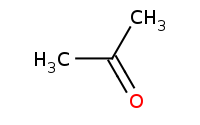

|

(catalyst)
 |
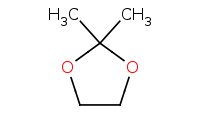

Note: Use of a diol can result in a cyclic acetal. Driving conditions are still necessary to drive the reaction equilibrium towards completion.
|
|
|
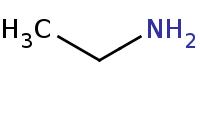

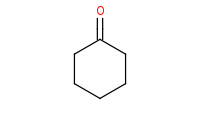

|

(catalyst)
 |
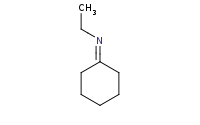

Note: Addition of a primary amine to a carbonyl yields a carbinolamine (hemiaminal) intermediate, but these are unstable and will dehydrate to form imines under any mildly acidic conditions. Note that the acid catalyst must be mild or else it will simply protonate the amine base in the first place, preventing its use as a nucleophile.
|
|
|



|

(catalyst)
 |
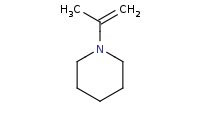

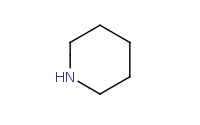
Note: Addition of a secondary amine (such as this cyclic amine) to a carbonyl is similar to addition of a primary amine, but there are not enough hydrogens on the amine to lose to form an imine. As a result, deprotonation will instead occur at an α carbon to yield an enamine upon dehydration.
|
|
|

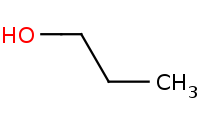

|

(catalyst)
 |
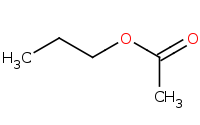

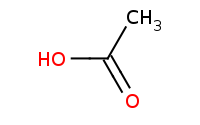
Note: Acid-catalyzed Fischer esterification is the only direct acylation reaction that works for carboxylic acids.
|
|
|
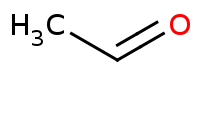

|

(catalyst)
 |
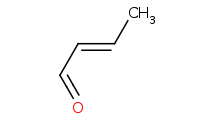

Note: Acid-catalyzed aldol reaction via an enol intermediate. Under acidic conditions, the dehydration (aldol condensation) product will be preferred over the beta hydroxy (aldol addition) product, helped by the conjugation of the alpha,beta unsaturated system.
|
|
|


|

(catalyst)
 |
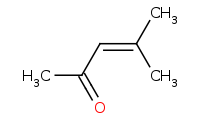

Note: Acid-catalyzed aldol condensation of a ketone.
|
|
|


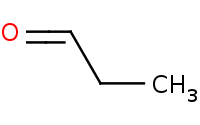

|

(catalyst)
 |


Warning: Crossed aldol reactions will generally be very messy (4 product classes) unless the reactants are carefully chosen.
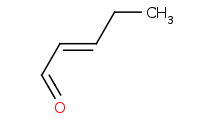

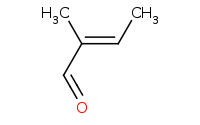

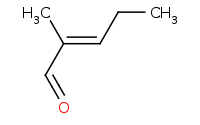

|
|
|
![C[C@H]1CO1](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=C%5BC%40H%5D1CO1&width=200&height=125&arrowdesc=&extraImageSetting=amap)



|

(catalyst)
 |
CO](https://re.edugen.wiley.com/arrow-webapp/ArrowWebService?action=smi2png&smiles=CCO%5BC%40H%5D%28C%29CO&width=200&height=125&arrowdesc=&extraImageSetting=amap)

Note: Acid-catalyzed epoxide opening. Note regioselective preference for more substituted side. Similar to Sn1/E1 selectivity, but no actual carbocation formed, thus still stereospecific
|
|
|
|
|
|
(0.062 sec)
Link
|
|